How To Remove Rust From Galvanized Metal
As outdoor metal surfaces age, they are exposed to the elements and start to corrode. The most mutual blazon of corrosion seen on galvanized steel is zinc rust. This post will discuss why this occurs, what types of metallic coatings are bachelor for protecting galvanized steel from corrosion and which type you may desire to choose for your application.
How does zinc prevent rusting?
Zinc is a metal that can be surprisingly resistant to rusting. This may audio counterintuitive since zinc oxide (the most mutual form of corrosion) is white and the well-nigh visible sign of oxidation on galvanized steel surfaces. What many people don't know about this phenomenon is that it happens considering zinc has an affinity for water molecules, which means they are more likely to stick around than with other metals like iron or aluminum. When they do come in contact with air, these water molecules react together and create a protective layer called hydroxyls on the surface of the metal.
CRC 05048 Zinc-It Instant Common cold Galvanize
Zinc-It Instant Cold Galvanize is the fastest drying, highest performance zinc coating available. It dries to a flat gray finish in less than five minutes and provides active rust protection for up to half-dozen months. Zinc-It's advanced formula is an excellent culling to hot-dip galvanization that provides on-the-task convenience without sacrificing quality or durability.

- Dries fast.
- High-Performance coating of >93% pure zinc. Actively fights rust and corrosion. Leaves a flat grayness paintable finish on surfaces.
How fast does zinc rust?
It depends on the surround and the type of coating. Zinc is more resistant to rusting in a marine atmosphere considering information technology'south less humid, whereas boosted humidity can speed upward corrosion rates on galvanized steel.
Because zinc has an analogousness for water molecules, hydrated zinc (zinc that has been contaminated past exposure to moisture) forms faster than dry out oxide, though this process takes typically 18 months or so in most cases earlier there are noticeable signs of deterioration visible through visual inspection lonely.

There are many different types of coatings bachelor today with varying levels of effectiveness against oxidation and other factors similar temperature modify. Electroless nickel plating is probably one of the best choices.
Does zinc rust in saltwater or air?
Saltwater is an aggressive and corrosive environment that presents a high adventure of corrosion to unprotected steel.
Zinc is not resistant to saltwater environments, then the zinc blanket will corrode over time and expose the underlying metallic or substrate textile.
Air corrosion occurs when wet enters an area where it would normally be dry out, such as in littoral regions with salty air. In this example, galvanized steel without protective coatings may feel surface rusting within simply hours after being exposed to moist air for extended periods of fourth dimension.
The most prevalent blazon of oxidation on outside galvanized steel surfaces is scissure corrosion which typically starts near cracks or other precipitous edges like rivets before spreading outwards from there.
Does zinc rust in concrete?
Zinc does non oxidize in concrete.
Does zinc rust in water?
Well, information technology depends on some factors such as the type of zinc, the pH level, and other factors.
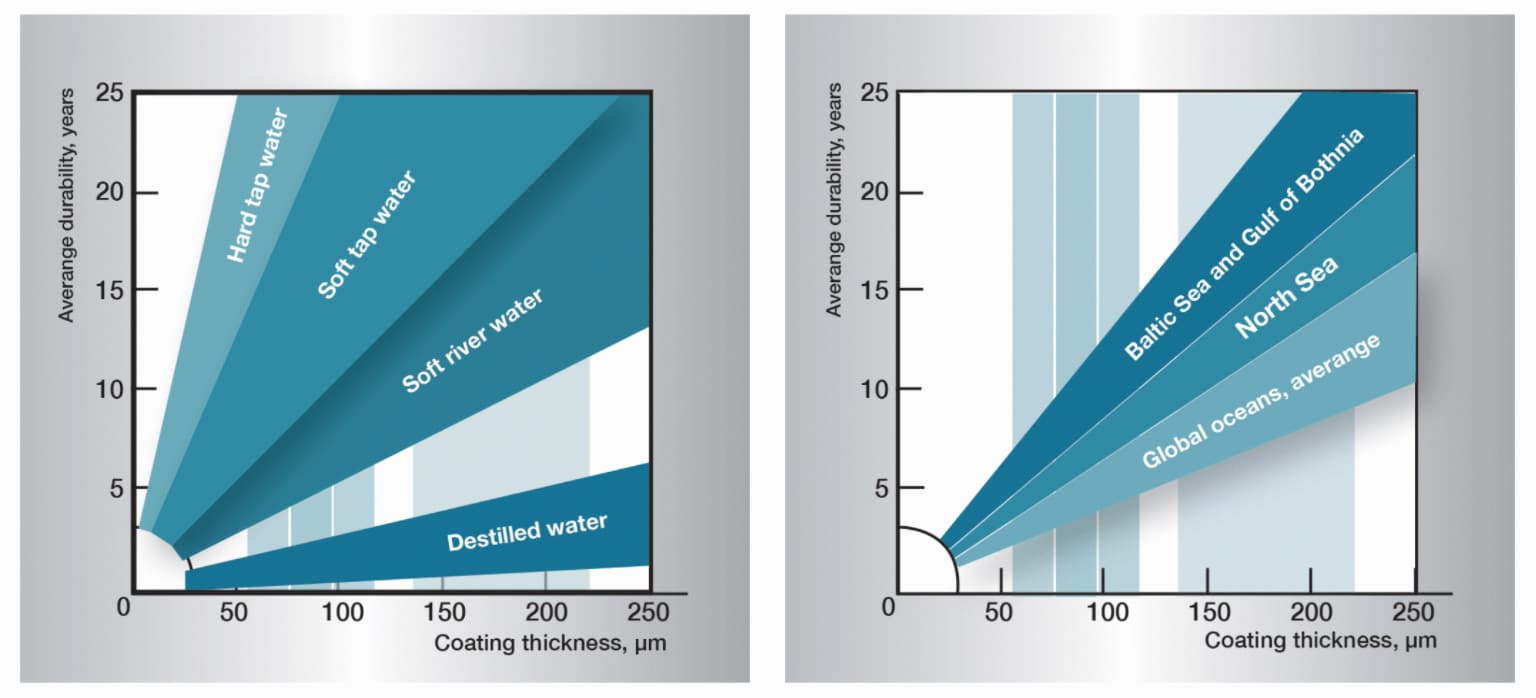
Zinc does not typically rust in h2o unless it is exposed to high acerbity or depression alkalinity levels which tin cause corrosion over time.
How does zinc blanket forestall rusting?
As mentioned above, the galvanized layer of zinc is an excellent bulwark against corrosion.
Zinc blanket prevents rusting by creating a tough, protective outer shell that slows downwards oxidation and protects the steel from exposure to moisture.

Steel coated with zinc does not need whatever other type of metal plating or pigment because information technology provides protection in all conditions for years.
In Conclusion
The respond to the question "does zinc rust" is yes, information technology does. Nevertheless, unlike atomic number 26 and steel which react with oxygen in the air to class a layer of oxide on their surface that helps protect them from further corrosion, zinc'southward low reactivity ways that oxidation doesn't happen very speedily or fully. Zinc too reacts poorly with h2o then if you do detect yourself in an emergency state of affairs where your metallic has come into contact with both water and air for some time then it'south best to remove information technology as soon every bit possible earlier any more damage can be done.
Source: https://rustconverters.net/does-zinc-rust/
Posted by: fergusonronage.blogspot.com


0 Response to "How To Remove Rust From Galvanized Metal"
Post a Comment